Abstract
Introduction:
In acute lymphoblastic leukemia (ALL) adolescents and young adults (AYA), there are benefits when using pediatric over adult treatment regimes. There is improved overall survival (OS), progression free survival (PFS) and reduced need for hematopoietic stem cell transplantation; all while having an acceptable toxicity profile. Yet, studies supporting this were largely performed in a western population (e.g., United Kingdom and United States) and thus may have had limited representation of the Asian population. Whether these results are generalizable and applicable to an Asian population is in question. MASPORE, a locally designed pediatric regiment based on BFM regimen, which has been used successfully in Singapore and Malaysia's AYA cohort since 2007. MASPORE protocol utilized PCR-based minimal residual disease (MRD) marker to risk stratify patients according to disease severity and used a 3- or 4-drug induction regimen depending on intermediate or high-risk stratification (L-asparaginase, Vincristine, Dexamethasone +/- daunorubicin). In contrast HyperCVAD does not use MRD to stratify or guide management. In this retrospective study performed in Singapore, we aim to demonstrate that MASPORE is an effective treatment option for AYA ALL in Asian population.
Methods:
Patient registries (IRB approved) spanning from January 2005 to June 2021 from three tertiary hospitals in Singapore were reviewed. ALL patients who were AYA, defined as less than 40 years of age, treated during this time were included and analyzed. Patients were treated with either the pediatric protocol MASPORE or with the adult protocol HyperCVAD. Patients who are Philadelphia positive were excluded from the MASPORE arm up to 2020. Patients' demographics, functional status, risk profile, adverse events, as well as the hematological response and transplant status were collected and analyzed with Pearson chi-square test. Kaplan Meier curve analysis were performed for OS and PFS with SPSS software.
Results:
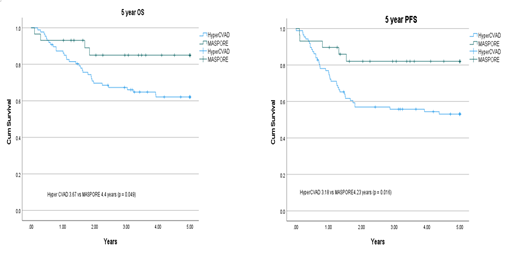
In this retrospective study, 116 patients were analyzed. Among these patients 29 (25%) received MASPORE and 87 (75%) received HyperCVAD. The median age was 23 (range 18-40) for MASPORE arm vs 28 years (range 15-40) for the HCVAD arm. median follow-up time was 4.5 vs 10.8 years. Both groups were similar in gender, race, ECOG status, and ALL risk status at diagnosis. Likewise, both arms did not have differences in starting hematological parameters: hemoglobin, total white blood cell count, platelet count, absolute neutrophil count, and bone marrow blast percentages. Median time from diagnosis to treatment was 3 and 4 days for MASPORE and HyperCVAD arms respectively. There were increased adverse rates for patients in the MASPORE arm. They had more (13.8 vs 0.0%, p < 0.001) of which 75% of the pancreatitis were CTCAE grade 3/4, avascular necrosis (13.8 vs 2.2%, p = 0.016), cerebral venous thrombosis (13.8 vs 1.1%, p = 0.004) and other thrombosis (27.6 vs 5.7%, p = 0.004). For the MASPORE and HyperCVAD arms there was one induction death, one from sepsis and the other from pneumonia . Both arms managed to achieve complete response (CR) with equivalent rates (82.5 vs 80.5%, p = 0.752). However, there was less relapsed or refractory disease in those treated with MASPORE (10.3 vs 44.8%, p < 0.001). The 5- OS was better in the MASPORE versus the HyperCVAD arm (4.4 vs 3.7 years; p = 0.049). Likewise, the 5-year PFS was superior in the MASPORE arm (4.2 vs 3.2 years, p = 0.034). Furthermore, a smaller proportion of patients required consolidative stem cell transplant (13.8 vs 49.4%, p < 0.001).
Conclusion:
The pediatric-inspired protocol MASPORE achieved similar CR rates with OS and PFS benefit, reduced relapse rates, and less need for consolidative stem cell transplant. MASPORE serves as a viable treatment option when treating AYA patients with ALL. Larger prospective studies are needed to further explore its use.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal